american academy of pediatrics covid vaccine under 12
The Centers for Disease Control and Prevention CDC director signed off on boosters for this group Wednesday after an advisory group voted 13-1 in favor and called for a strong recommendation. Skip to the content.

As School Openings Stir Covid 19 Outbreaks Pediatricians Sound The Alarm Aamc
After months of waiting COVID vaccines for children between 5 and 11 years of age are available.
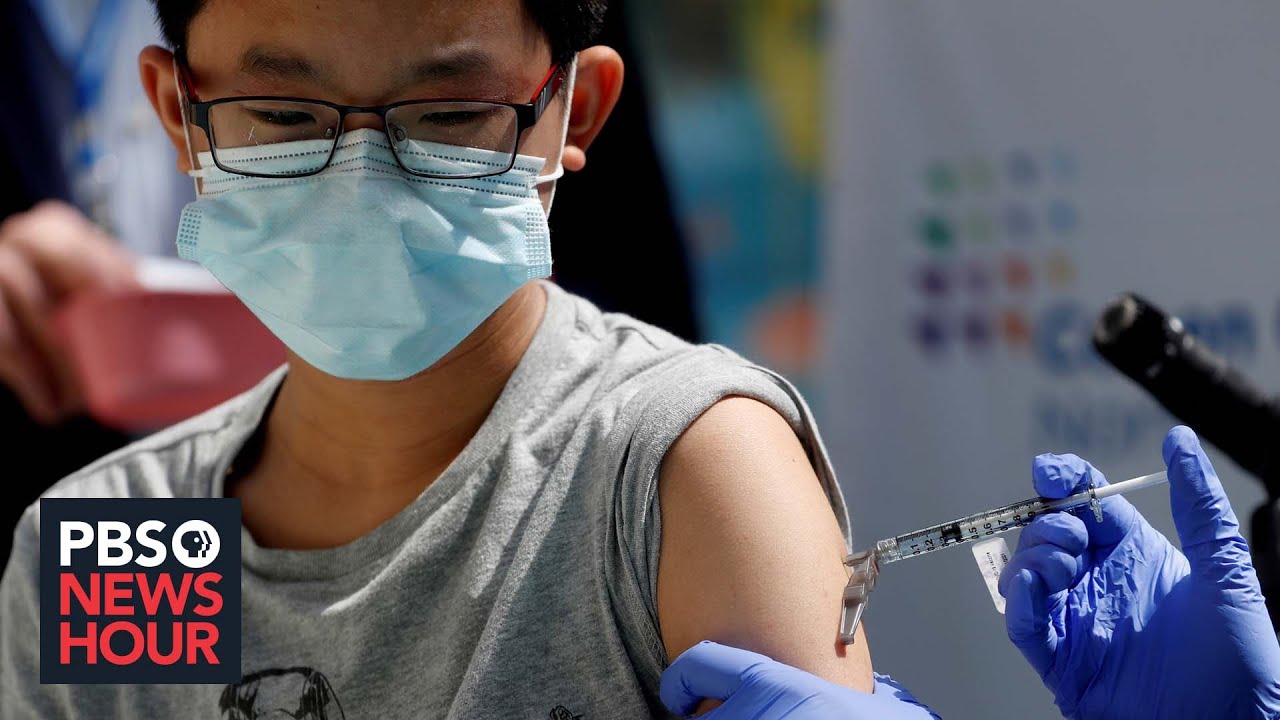
. AAP CDC recommend COVID-19 vaccine for ages 12 and older May 12 2021 In addition to approving the Pfizer-BioNTech vaccine for adolescents the CDC updated its clinical guidance to allow coadministration of vaccines. Josh wiglesworth said his office received 600 doses of the vaccine and began. The Pfizer vaccine dosage for.
For adolescents and young adults the risk of myocarditis caused by covid infection is much higher than after mrna vaccination. NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID-19 vaccines for children under 12. Only the Pfizer-BioNTech Covid vaccine has been authorized for adolescents and teens ages 12-17.
American academy of pediatrics covid vaccine 5-11. ITASCA IL -- Today the Food and Drug Administration FDA finalized the full approval of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older. While the Covid vaccines in use in.
Adolescents 12-15 receive 2 doses of the adult formulation where as children. FDA authorizes COVID-19 vaccine for adolescents ages 12-15. The vaccine was found to be 907 effective in children ages 5-11 and 100 effective in children ages 12- 15The COVID-19.
Currently only the Pfizer COVID-19 vaccine has an emergency use authorization for children 12 and up. Because theyre still growing and developing its critical that these vaccines are evaluated in well-designed and well-conducted clinical trials Children under 5 years would receive doses of 3 micrograms µg. In a new policy statement the American Academy of Pediatrics AAP recommends vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
The AAP policy statement comes amid a discussion by the Advisory Committee on Immunization Practices of the Centers for Disease Control and. How children can get vaccinated through ui health 2 the white house said wednesday. Making more vaccines tests and treatments available to all.
Adolescents ages 12 years and older receive the same dose of Pfizer-BioNTech COVID-19 vaccine as adults. American Academy of Pediatrics urges FDA to approve COVID vaccines for children under 12 Sep 13 2021 640 PM EST. Home Unlabelled American Academy Of Pediatrics Covid Vaccine Breastfeeding Buff Dudes Dumbbell workout Pdf free 12 w eek dumbbel l only plan - The american college of obstetricians and gynecologists acog is.
Saturday March 5th 2022. For adolescents and young adults the risk of myocarditis caused by covid infection is much higher. As Covid cases among children continue to rise in the US due to spread of the Delta variant experts are urging the federal government to fast-track vaccine approval for those under the age of 12.
The american academy of pediatrics. Many are wondering about safety said dr. Unlike many medications COVID-19 vaccine dosage does not vary by patient weight but by age on the day of vaccination.
American Academy of Pediatrics Cautions Against Off-Label Use of COVID-19 Vaccines in Children Under 12. It is a lower dose than the 10-µg doses used for children ages 5-11 years and 30-µg doses used for those 12 years and older. The American Academy of Pediatrics is urging the Food and Drug Administration to approve a COVID-19 vaccine for children under the age of 12 as soon as possible.
Adolescents ages 12-15 years can begin receiving COVID-19 vaccine boosters. A single booster dose of the Pfizer vaccine is authorized for kids 12 through 17 years old at least 5 months after the second dose of vaccine. While questions may now arise about the administration of the vaccine off-label for children aged 11.
Though many parents started making appointments weeks. White House COVID plan. Tuesday February 8 2022.
The American Academy of Pediatrics recommends COVID-19 vaccines for all eligible children 5 years and olderMillions of kids and teens already have been safely vaccinatedVaccination protects your child from becoming seriously ill or dying from COVID. The American Academy of Pediatrics AAP reported that since the beginning of the pandemic about 106 million children have been infected with COVID-19 which is about 18 of all US. Ensuring vaccines are widely available for children under 5 years once a vaccine is authorized.
They can reduce the risk of severe illness from COVID. Among states reporting children ranged from 17 to 44 of their total cumulated hospitalizations. 2 the CDC endorsed the recommendation to extend eligibility for the Pfizer vaccine to children between 5 and 11 years of age and urged parents to get their children vaccinated as soon as they can.
Children ages 5 through 11 years receive an age-appropriate dose of the Pfizer-BioNTech COVID-19 vaccine. While 70 of eligible Mainers 12. The vaccines cannot give you COVID.
The Pfizer COVID-19 vaccine has been given FDA Emergency Use Authorization for children over the age of five while Moderna and Johnson Johnsons COVID vaccines are approved for use in adolescents over age 18. The White House released its latest COVID-19 plan on Wednesday to account for the changing dynamics of the pandemic. American Academy Of Pediatrics Covid Vaccine Myocarditis.
Other vaccines likely wont be far behind. The viral vector vaccine in the United States is given in one dose to people 18 years and older. Ad Safety is CDCs top priority and vaccination is the safest way to help build protection.
Currently the Pfizer-BioNTech mRNA vaccine has been authorizedrecommended for children and adolescents aged 5-15. Neither the Johnson Johnson or Moderna vaccine have been approved for anyone under age 18. American Academy of Pediatrics Oregon Vaccine News from covidblogoregongov the observed risk is highest in young males age 12 to 29 but covid infection can also cause myocarditis she pointed out.

Covid 19 Vaccine For Kids Under 12 What Parents Need To Know University Hospitals
Covid Fda Plan To Fast Track Pfizer Vaccine For Kids Under 5 Fails

Fda Again Warns Parents Not To Get Children Under 12 Vaccinated Yet The New York Times

American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube

What To Know As Covid Vaccines For Kids Under 12 Face Final Hurdles Nbc Chicago
Parents And Pediatricians Are Growing Impatient For A Covid 19 Vaccine For Younger Children Cnn

Fda Authorizes Covid Vaccine Booster Shots For Kids Aged 12 To 15 People Com

Q A Aap Endorses Giving Routine Immunizations Covid 19 Vaccines At Same Time
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says
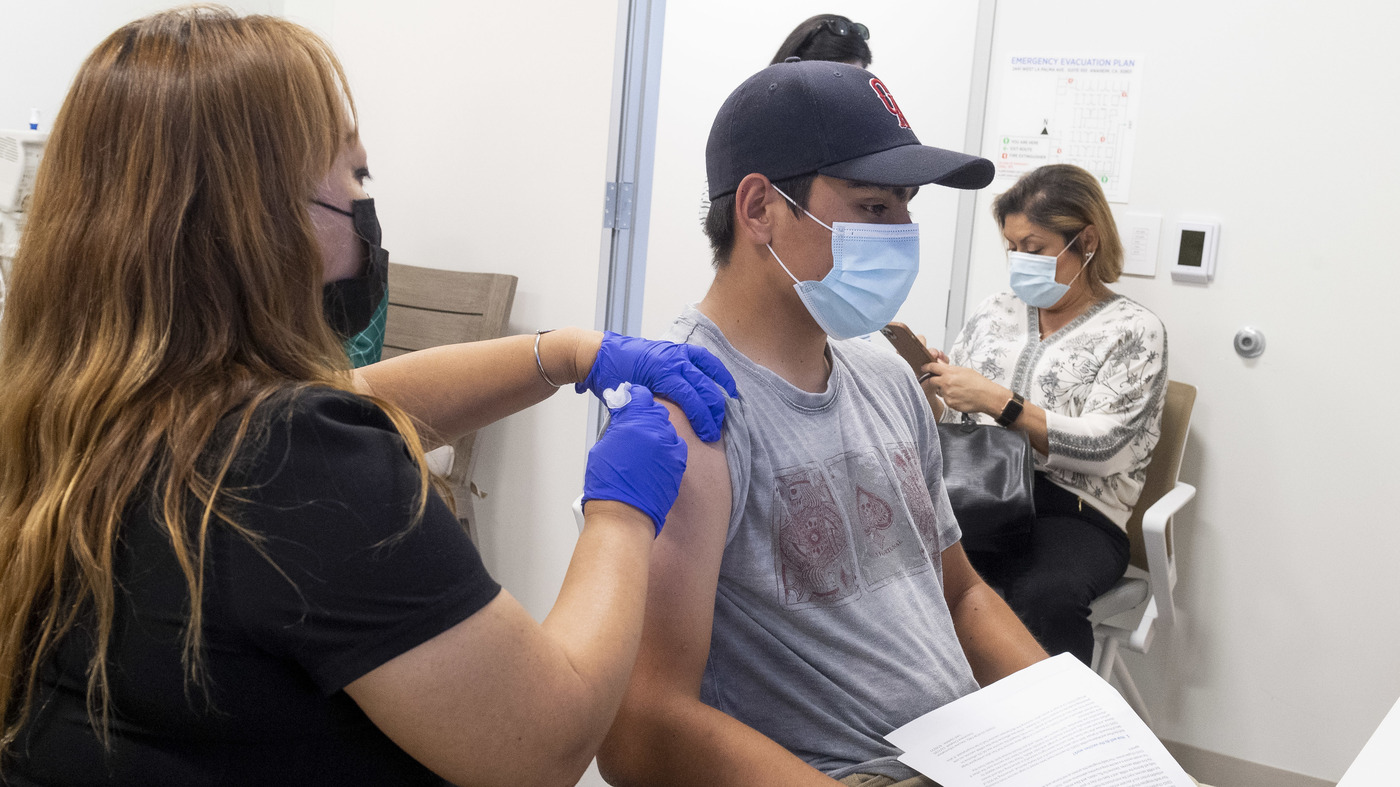
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr

As Omicron Surges Effort To Vaccinate Young Children Stalls Kaiser Health News

What Is Happening With Covid 19 Vaccines For Kids Under 12 Minnpost

Faq Covid 19 Vaccine For Kids Under 12 Lurie Children S

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
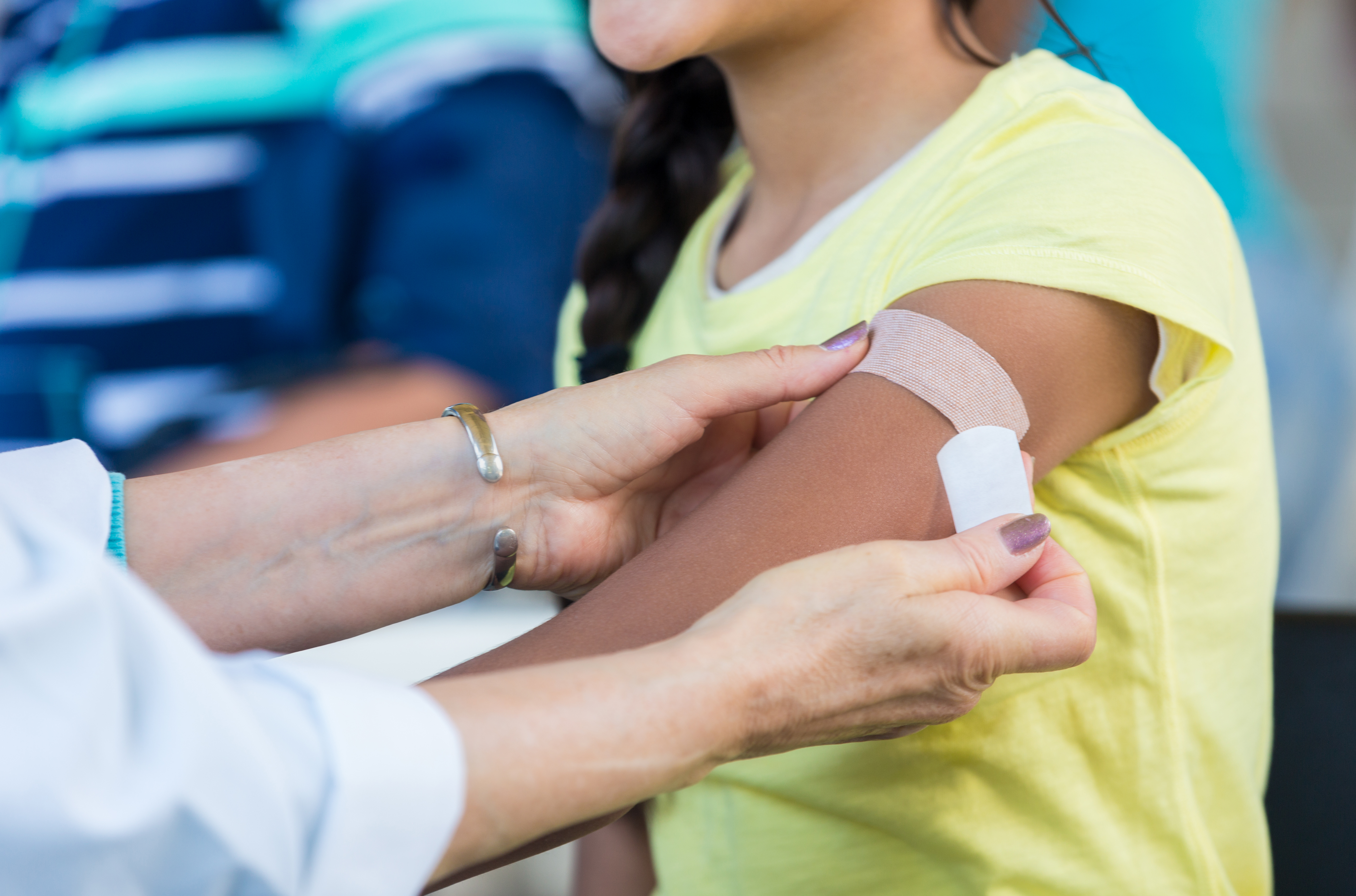
Pfizer Gets Fda Approval To Enroll Kids As Young As 12 In Covid 19 Vaccine Trial Shots Health News Npr
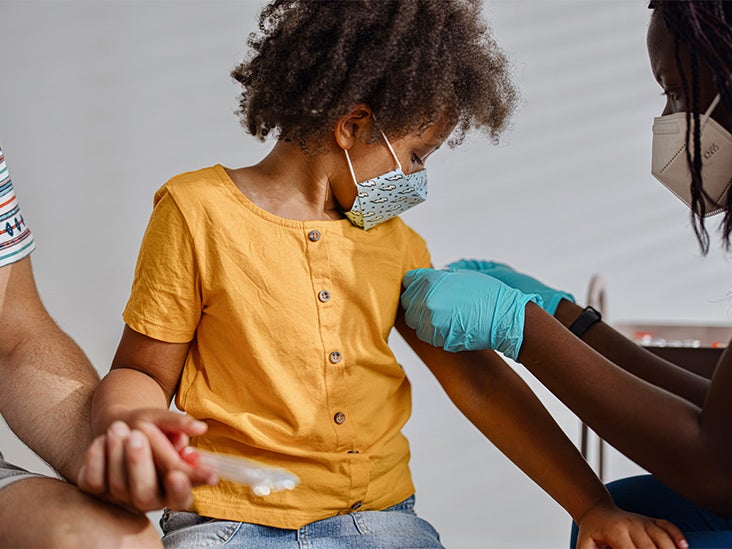
What Parents Should Know About Covid 19 Vaccines For Kids Under 12
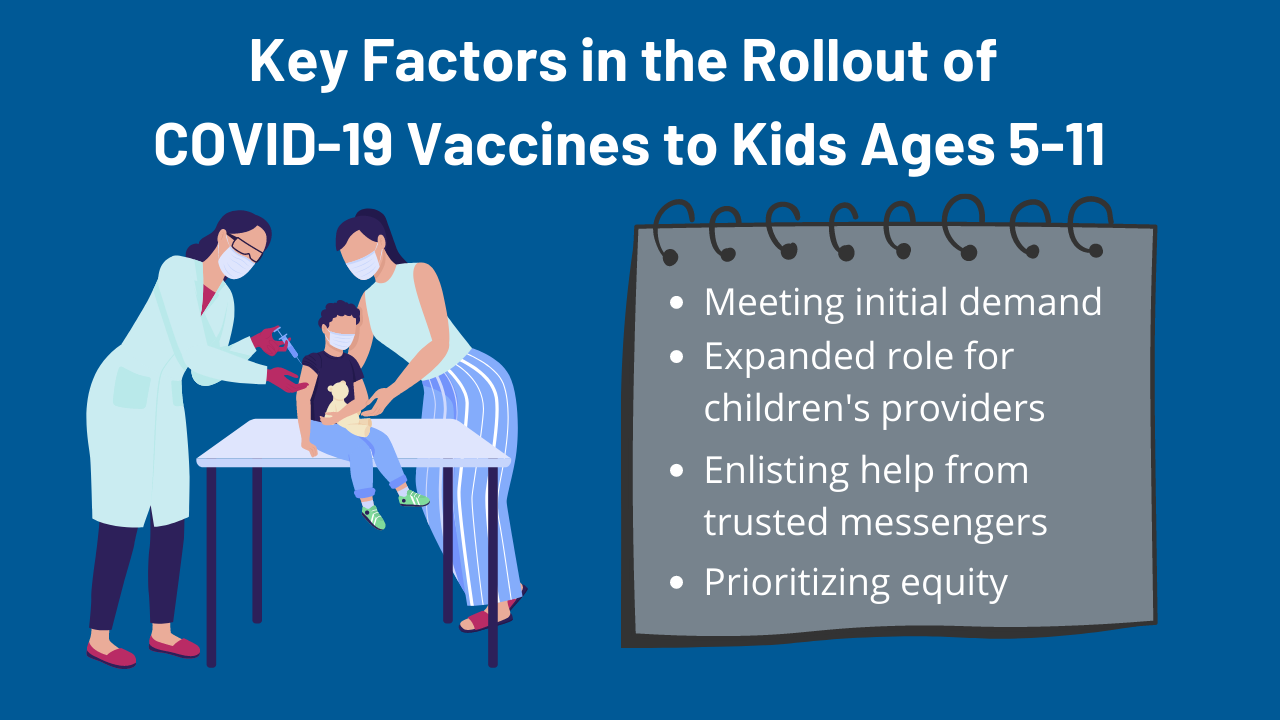
Vaccinating Children Ages 5 11 Policy Considerations For Covid 19 Vaccine Rollout Kff

Fact Check How Useful Are Coronavirus Vaccines For Children And Adolescents Coronavirus And Covid 19 Latest News About Covid 19 Dw 06 08 2021

Biden Covid Vaccine Plan For Kids Under 12 What To Know Los Angeles Times